- Home
- Faculty Members
- Organization for Fundamental Education
- Hiroyuki Kurata
Organization for Fundamental Education
- Key words
- Structural organic chemistry, organic synthesis, extended π-electronic systems, functional organic materials, organic emitting materials

Doctor of Science / Professor
Hiroyuki Kurata
Education
Graduate School of Science, Osaka University (Doctor Program)
Professional Background
Research associate at Osaka University, researcher at Georg-August Universität Göttingen, assistant professor at Osaka University, lecturer at Osaka University
Consultations, Lectures, and Collaborative Research Themes
Consultation on the synthesis and properties of oraganic materials (low-molecular compounds). Lectures on chemical substances around us.
Main research themes and their characteristics
「Synthesis of Nonplanar Extended Quinone Molecules Based on Benzofused Rings and Evaluation of Their Responsiveness to External Stimuli」
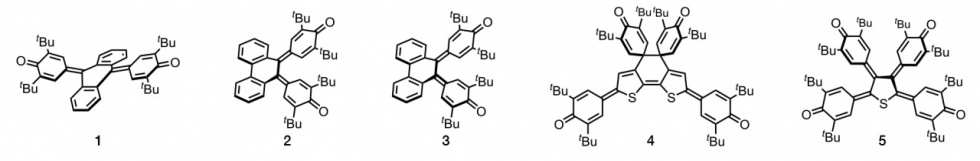
Extended quinone is a generic term for quinones with two carbonyl groups attached to different rings, and is a term used in structural organic chemistry. There are two types of structures inherent in this compound: quinone and biradical structures. The planarity of the molecule as well as the structure and properties of the π-electron spacer have a great influence on the contribution of the two structures. However, if the molecule is non-planarized, an energy barrier is created between the two structures, and both structures are stimulated by an external stimulus. Therefore, we introduced the concept of "non-planarization by benzoannelated ring", and we have developed a dibenzoannelated p-terphenoquinone (1). Since the discovery of photo-irradiated switching function of the quinone and biradical structures of 1, we have been investigating various non-planar extended quinones (2, 3), which have a switching function for light and heat.
During the study, a novel structure, bis(spirodzienone)-bridged bithiophene, was discovered, and this structure exhibited a response to external stimuli based on bond breaking-recombination (4). A wide variety of responses were observed depending on the fusing mode of the thiophene ring and the type of substituents. We have found that some crystals showed the significant color change caused by external mechanical forces, which is called “mechanochromism”.
We have also synthesized extended quinones (5), which are highly non-planarized by introducing quinomethyde structures into all the CH moieties of the thiophene ring, and found that they exhibit unique redox behavior and electrochromism.
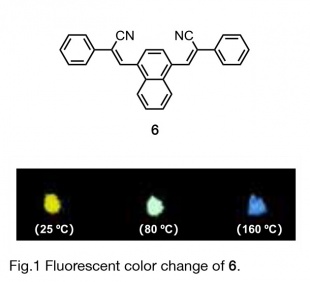
「Creation of luminescent organic solids by utilizing the characteristics of fused π-electron systems」
In general, most luminescent organic compounds emit light in solution but quench in the solid state. Recently, however, some luminescent compounds have been discovered that emit light only in the solid state. This phenomenon is called "Aggregation-Induced Emission (AIE)”, which has attracted much attention from the viewpoint of organic luminescence chemistry. The arrangement of the molecules in the crystal plays an important role in the development of solid-state luminescence, and it is known that the mother skeleton is a solid. The arrangement of molecules in the crystal plays an important role in the development of solidstate luminescence, and even if the mother skeleton does not have solid-state luminescent properties, novel luminescent organic solids can be obtained by appropriate interactions between molecules in the crystal by molecular modification such as benzoannelated rings. We are systematically synthesizing various luminescent molecules fused with various π-electron systems in order to elucidate the changes in the properties and functions of the generated luminescent organic solids.
Major academic publications
H. Kurata, T. Inoue, T. Suzuki, Y. Hirao, K. Matsumoto, T. Kubo, “Synthesis, Structure, and Properties of Quinone Methides Incorporating Thiophene and Bithiophene Derivatives: New Overcrowded Extended Quinonoid π-Systems” Synlett, 2016, 27(14), 2133−2139.
K. Adachi, Y. Hirao, K. Matsumoto, T. Kubo, H. Kurata, “Synthesis of Sexithiophene-Bridged Cage Compound: A New Class of Three-Dimensionally Expanded Oligothiophenes”, Organic Letters 2014, 16(22), 5870−5873.
“Cross Conjugation − Modern Dendralene, Radialene and Fulvene Chemistry” (Ed. H. Hopf, M. S. Sherburn), Wiley−VCH, 2016. 共著.